Sodium Hydroxide Reacts With Nitric Acid Balanced Equation
Write a balanced chemical equation showing the reaction between nitric acid and sodium hydroxide. Nitric acid Sodium.

How To Balance Hno3 Naoh Nano3 H2o Nitric Acid Plus Sodium Hydroxide Youtube
In this video well balance the equation NH4OH HNO3 NH4NO3 H2O and provide the correct coefficients for each compoundTo balance NH4OH HNO3 NH4NO3.

. Generally it is double replacement but a. For this reaction we have a neutralization reaction. KNO3 is the salt in the above reaction.
Nitric acid Sodium hydroxide Sodium nitrate Water. Hydrochloric acid is an acid because it has hydrogen ionThe formula for hydrochloric acid is HClIt is a strong acid. An acetic acid buffer solution is required.
Heat energy is generally released and the amount of heat. HNO_3 NaOH NaNO_3 H_2O The two reactants react in a one to one ratio. HNO3 NaOH --.
NaNO3 H20 Or. Reaction between sodium hydroxide and nitric acid is a textbook example of acid base neutralisation. NaOH HNO 3 NaNO 3 H 2 O.
Sodium hydroxide reacts with nitric acid NaOHaq HNO3aq -- H2Ol NaNO3aq Write balanced chemical equation for the double-replacement reactions that occur in aqueous solution. 2 moles of Sodium Hydroxide react with 1 mole of Sulfuric Acid to form 1 mole of Sodium Sulfate and 2 moles of Water. By Mar 20 2021 Non classé Mar 20 2021 Non classé.
Acid Base -- Salt Water Therefore. What special kind of reaction is this. Nitric acid reacts with sodium hydroxide to produce a salt and water.
Salt Water Therefore. Nitric acid is a relatively strong acid meaning it tends to ionize almost completely in aqueous solution. One molecule of Nitric acid HNO_3 is neutralized by one molecule of sodium hydroxide NaOH.
The reaction of Nitric acid and Sodium hydroxide represents a net ionic equation involving a strong acid and strong base. A beaker of nitric acid is neutralized with calcium hydroxide. NaOH base This is therefore an acid-base reaction.
A Write a complete balanced equation with physical states 7 points b How many mL of 0050 M nitric acid is. Sodium Hydroxide Sulfuric Acid Sodium Sulfate Water. Sodium ions combine with nitrate ions to form sodium nitrite and hydrogen ions.
This means that we will split them apart in the net ionic. Strong acids and strong bases are considered strong electrolytes and will dissociate completely. HNO3 NaOH -- NaNO3 H20 Or.
Is nitric acid a base or an acid. HNO3 acid Sodium hydroxide. Acid Base --.
Nitric acid sodium hydroxide balanced equation. HNO3 KOH H2O KNO3 Neutralization produces a salt and water. Give balanced equationa Dilute sulphuric acid reacts with Aluminium powderb Potassium carbonate reacts with hydrochloric acidc Potassium hydroxide reacts with nitric acidd Zinc.
Write a balanced molecular equation and a net ionic equation for his reaction. Type of Chemical Reaction. The equation balanced by program.
H2 acts as a base to abstract an H from the nitric. HNO3 acid Sodium hydroxide. NaOH base This is therefore an acid-base reaction.
Nitric acid Sodium. Hydrochloric Acid and Sodium Hydroxide Helloyou.

Chemical Reactions Copper Reactions Ppt Download
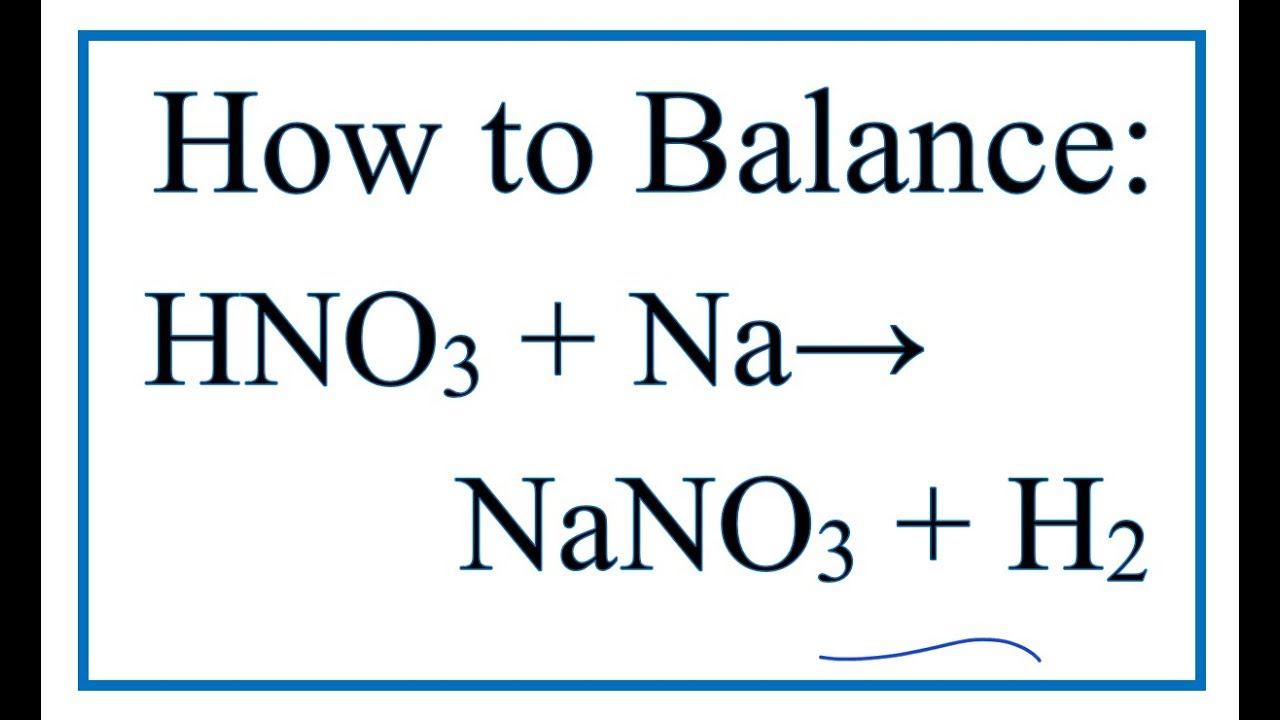
How To Balance Hno3 Na Nano3 H2 See Note In Description Youtube

Type Of Reaction For Hno3 Naoh Nano3 H2o Youtube

Ionic Equations A Chemical Equation Shows The Number Of Atoms And Molecules Of The Reactants And Products Also Shows Physical State Of Reactants And Products Ppt Download
Comments
Post a Comment